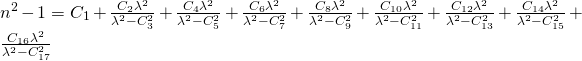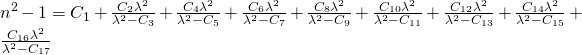(from latin dispergere, “to scatter”, to disperse” ) :
Dependency of a measure on frequency / wavelength.
 (C) Wikipedia, zum Animieren bitte klicken
(C) Wikipedia, zum Animieren bitte klicken
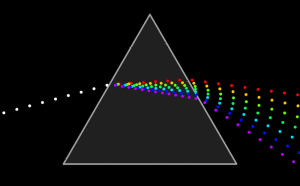
Using a Prism dispersion leads to splitting of white light beam into individual colors. A rainbow where light takes different paths inside the water dropplets, depending on their wavelength is another “real world” example of dispersion.
Every optical medium / glass type has different refraction indices for the various wavelength of light. The number that describes how different the light paths of the various wavelengths are, is the Abbe-number.
UNder dispersion formulas you find the most common formulas